
AstraZeneca at ACR 2023: Micki Hultquist Shares Post-hoc Analysis of Saphnelo
Shots:
-
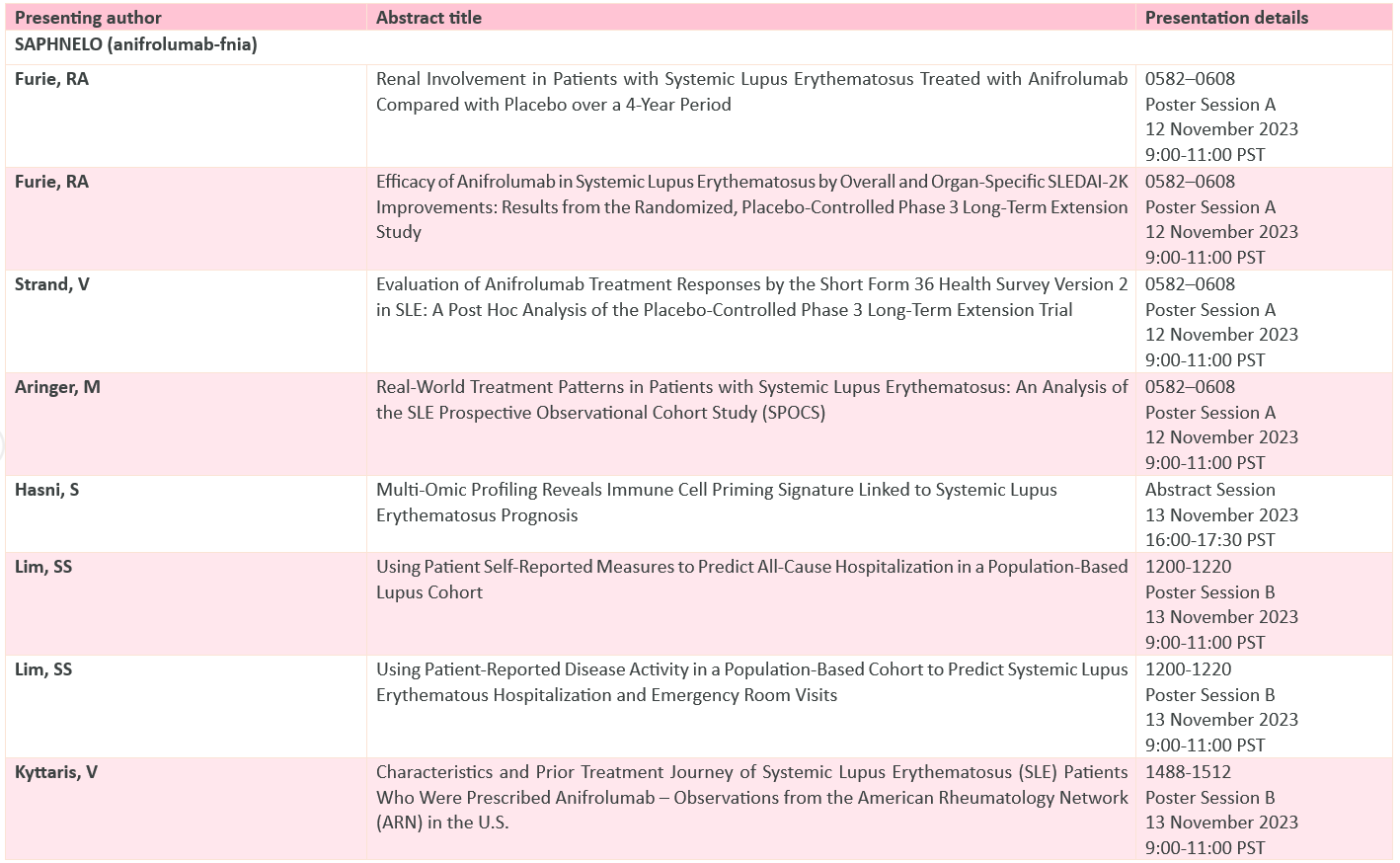
AstraZeneca at ACR 2023 presented 18 abstracts from its respiratory and immunology pipelines
-
Micki Hultquist in a stimulating conversation with PharmaShots sheds light on Saphnelo, AstraZeneca’s first-in-class biologic treatment for systemic lupus erythematosus (SLE)
-
The post-hoc analysis of the TULIP P-III program reveals that the patients showcased more frequent and sustained remission as compared to standard therapy is among 13 publications related Saphnelo
Saurabh: As the company has already planned to present 18 abstracts from its respiratory and immunology pipeline, could you brief us all about it?
Micki: We are really excited about the strong presence of AstraZeneca at ACR this year. We’re presenting data across our portfolio, including from Saphnelo, our first-in-class biologic treatment for systemic lupus erythematosus (SLE), as well as late-breaking full results from the Phase 3 MANDARA trial in EGPA from Fasenra.
Importantly, a new post-hoc analysis of the TULIP Phase III program shows that Saphnelo, a first-in-class biologic, is associated with more frequent and sustained remission versus standard therapy alone. These data are important because SLE is a serious and complex autoimmune condition that can affect any organ. Patients often experience inadequate disease control, and despite advances in therapies, feMaw experience remission.
With Saphnelo, and across our portfolio in Immunology, we remain focused on our ambition to challenge current treatment expectations and help establish remission as an achievable treatment goal for as many patients as possible.
Full abstract list below:
Saurabh: As we all heard the P-III (TULIP) program will be presented at ACR 2023, give us more information regarding the study & its design?
Micki: This new post-hoc analysis of the TULIP Phase III program provides evidence across four years that remission is an achievable goal with Saphnelo (anifrolumab), a first-in-class biologic for patients living with systemic lupus erythematosus (SLE). Saphnelo is the first biologic with remission data from a four-year placebo-controlled trial.
At the end of the four-year TULIP program (week 208), 33% (n=63/194) of patients treated with Saphnelo were in remission compared with 21.4% (n=14/65) on standard therapy alone. In addition, patients treated with Saphnelo spent a greater percentage of time in remission (17.2% vs. 8.3%; 95% CI 3.2-14.5; p=0.0022) and were more likely to sustain remission for three or more visits vs. standard therapy alone (30.2% vs. 17.2%; 95% CI 1.2-3.6; p=0.0127).
These data are exciting as they demonstrate that remission is an achievable therapeutic goal with Saphnelo. In lupus, remission is associated with reduced organ damage, fewer flares, reduced hospitalization, reduced mortality and improved health-related quality of life.
All three TULIP trials for Saphnelo (TULIP-1, TULIP-2, and TULIP-LTE, or long-term extension) were randomized, double-blinded, placebo-controlled trials in patients with moderate to severe SLE who were receiving standard therapy. The placebo arm of the trial included at least one of the following standard therapies: OCS, antimalarials and immunosuppressants (methotrexate, azathioprine or mycophenolate mofetil).
Saurabh: Remission is crucial to improve the quality of life for SLE patients. Let us know how the firm is going to challenge the existing framework and make remission a realistic objective?
Micki: Our ambition is to disrupt Immunology by achieving increased rates of remission in under-served diseases and bringing in a new generation of therapeutics, aiming to reverse and repair damage, treat patients earlier, modify the natural course of the disease, and one day potentially offer a cure.
In lupus, remission is associated with reduced organ damage, fewer flares, reduced hospitalization, reduced mortality and improved health-related quality of life.
The new post-hoc analysis of the TULIP Phase III program presented at ACR 2023 provides evidence across four years that remission is an achievable therapeutic goal with Saphnelo. In the analysis, remission was defined using the DORIS criteria (Definition of Remission in SLE): no disease activity assessed by two measures of disease activity across organ systems (total SLEDAI-2K, or “Systemic Lupus Erythematosus Disease Activity Index 2000” score, and the physician global assessment), oral corticosteroid (OCS) dose of <5 mg per day, and no use of restricted medicines.
Recent recommendations from the European Alliance of Associations for Rheumatology (EULAR) advise taking an OCS-sparing approach to the management of SLE, with early use of biologics to achieve remission or lower disease activity, preserve renal function, and reduce flares and organ damage.
With Saphnelo, and across our portfolio in Immunology, we remain focused on our ambition to challenge current treatment expectations and help establish remission as an achievable treatment goal for as many patients as possible.
Saurabh: Saphnelo was studied against the standard treatment alone, & and the result showed significant data regarding the remission, can you let us know the exact data of the study?
Micki: The TULIP-LTE study concluded in 2022. This post-hoc analysis of the TULIP program, presented at ACR 2023, shows that at the end of the four-year TULIP program (week 208), 33% (n=63/194) of patients treated with Saphnelo were in remission compared with 21.4% (n=14/65) on standard therapy alone.
In addition, patients treated with Saphnelo spent a greater percentage of time in remission (17.2% vs. 8.3%; 95% CI 3.2-14.5; p=0.0022) and were more likely to sustain remission for three or more visits vs. standard therapy alone (30.2% vs. 17.2%; 95% CI 1.2-3.6; p=0.0127).
These data are exciting as they demonstrate that remission is an achievable therapeutic goal with long-term Saphnelo use.
Saurabh: The study (TULIP) was done for an extensive four years, what was the study design?
Micki: TULIP-LTE investigated the long-term safety and tolerability of Saphnelo compared with placebo in 559 enrolled patients with moderate to severe SLE who had previously completed a Phase III study (TULIP-1 or -2) for an additional three years.
In the post-hoc analysis presented at ACR, 369 patients (anifrolumab 300 mg, n=257; placebo, n=112) who continued treatment in TULIP-LTE were analyzed for the 4-year TULIP+LTE period.
At four years, TULIP-LTE is the longest placebo-controlled clinical trial performed in SLE to date and supports the favorable benefit-risk profile of Saphnelo seen in previous trials.
Image Source: Canva
About the Author:

Micki Hultquist
Micki Hultquist oversees the global development program for Saphnelo, the first new treatment in over a decade (and only the 2nd in 60 years) for the debilitating autoimmune disease, systemic lupus erythematosus (SLE). Saphnelo (anifrolumab) is a first-in-class drug that targets the type I interferon pathway – known for decades to be central to the pathophysiology in lupus, but one that no other company has been able to successfully target. Dozens of other potentially viable therapeutics have failed in clinical trials in lupus, given the complexity of the disease. SLE can affect any organ in the body, has unpredictable flares in disease activity and manifests differently in each patient. SLE is generally treated with corticosteroids (which are effective, but have high toxicity) and immunosuppressants. In her role on the anifrolumab program (one she has held for over 10 years), Micki oversees all aspects of development of anifrolumab in SLE and a broader LCM program, including other forms of lupus and myositis. It is the first drug AstraZeneca has launched in Immunology within its Respiratory & Immunology portfolio.
Tags

Saurabh is a Senior Content Writer at PharmaShots. He is a voracious reader and follows the recent trends and innovations of life science companies diligently. His work at PharmaShots involves writing articles, editing content, and proofreading drafts. He has a knack for writing content that covers the Biotech, MedTech, Pharmaceutical, and Healthcare sectors.














